Aqueous Solutions Are Best Described as
It is also readily soluble in solutions of the caustic alkalis slightly soluble in aqueous ammonia solution and almost insoluble in sodium carbonate solution. There are variety of factors that impact CO2R activity and selectivity including the catalyst surface structure morphology composition the choice of electrolyte ions and pH.
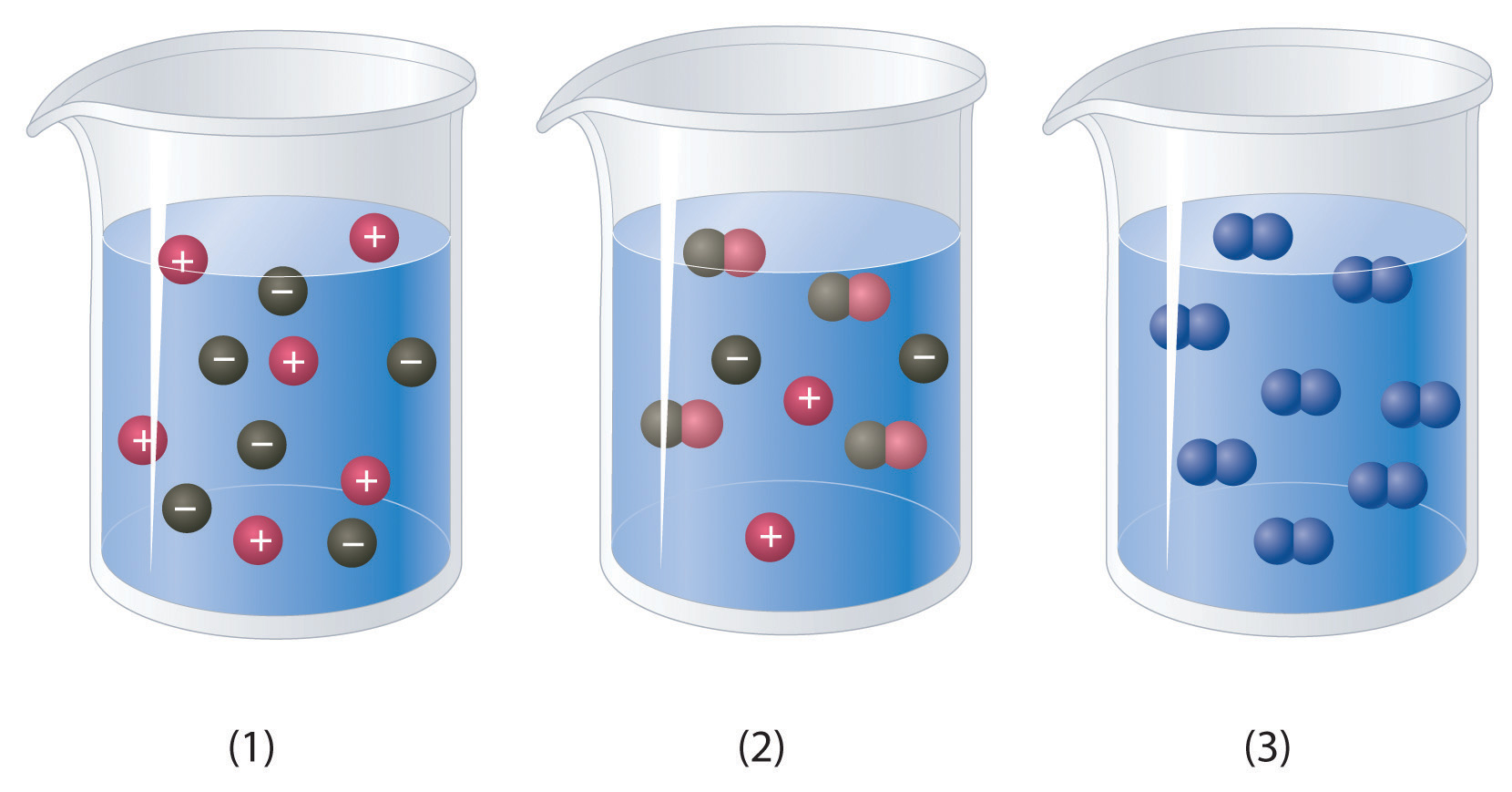
Ch104 Chapter 7 Solutions Chemistry
If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution.

. There are 5 standard solutions to prepare with your partner. Create a table in your notebook for your data. Stock solutions 1 mgml of each compound were made using 10 mM Tris pH 80 as the buffer.
The purpose of this research was to investigate the adsorption of arsenic As from aqueous solutions using. HEWL-buffer and NaBr-buffer solutions were mixed together in a 11 ratio just before the measurement and then transferred into black-walled quartz cuvettes with a path length of 1 cm and volume of 1 mL. The form of METHOCEL cellulose ether product chosen powder surface-treated powder or granules influences the techniques used to make solutions.
It requires calculating the number of moles of solute desired. Here the authors use a. Air for example is a solution.
Microkinetic analyses of aqueous electrochemistry involving gaseous H2 or O2 ie hydrogen evolution reaction HER hydrogen oxidation reaction HOR oxygen reduction reaction ORR and oxygen. NaBr-buffer solutions were filtered through 02 μm filter pores Sartorious and preheated between 40 and 50 C. The concentration of NADH and NADPH solutions was checked by absorbance at 340 nm using a Shimadzu UV-120-02 spectrophotometer.
Solutions of low-viscosity products can be made at 10 to 15 concentration while high-viscosity products have a normal limit at 2 to 3 concentration. Arrhenius acids and bases chemicals that interchange ceH with water to form ceH_3O or ceOH- break apart in water forming ionsIn your case there is an equilibrium ceHClaq H_2Ol H_3Oaq Cl-aq which very strongly lies to the right. CeHCll is not the correct notation in this case.
The procedure for preparing a solution of known concentration from a stock solution is shown in Figure 1213. Here we propose an electrolyte. MPAC-600 effectively removed 997 of AsV from aqueous solutions.
0 The metallic derivatives phenolates phenates or carbolates of the alkali metals are obtained by dissolving phenol in a solution of a caustic alkali in the absence of air. Regeneration and economic feasibility of the biosorbents were also assessed. The Langmuir isotherm best described the removal of AsIII using both biosorbents.
In Section 93 we described various ways of characterizing the concentration of. Solutions are all around us. Solution Beaker mass g Beaker salt g Beaker salt water g Calculated Salt massg Calculated Water mass g 1.
The final concentration of. To date copper is the only heterogeneous catalyst that has shown a propensity to produce valuable hydrocarbons and alcohols such as ethylene and ethanol from electrochemical CO2 reduction CO2R. Electrocatalytically reducing CO2 to ethanol can provide renewably generated fuel but catalysts are often poorly selective for this conversion.
Dilution is also used to prepare solutions from substances that are sold as concentrated aqueous solutions such as strong acids. Weigh out the mass of NaCl. The advantages and disadvantages of current physical and chemical treatment methods as described in the various sections so far are summarized in Table 1At present adsorption method is one of the widely used treatment methods because of its economic value effectiveness designoperation flexibility and potential reversibility Indeed adsorption is the efficient.
From Biosearch Bedford MA. Virtually all of the. Aqueous battery systems feature high safety but they usually suffer from low voltage and low energy density restricting their applications in large-scale storage.
You may split up the duties so that one of you makes 2 solutions and the other makes 3. Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. Further dilutions were then made using 10 mM Tris pH 80 as the diluent and 200 ml aliquots.

Aqueous Solutions Ck 12 Foundation

Aqueous Solutions Ck 12 Foundation


No comments for "Aqueous Solutions Are Best Described as"
Post a Comment